Abstract
Introduction: Ibrutinib is an oral once-daily Bruton's tyrosine kinase inhibitor (BTKi) and the only targeted treatment to demonstrate significant progression-free survival and overall survival benefit in multiple phase 3 trials for chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL). Additionally, efficacy outcomes for ibrutinib have been shown to be similar for patients with dose modifications due to adverse events compared to patients without dose modifications. Open-label studies with follow-on BTKi have suggested numerical differences in adverse event rates, however, whether these differences translate into real-world improvements in clinical outcomes remains unexplored. The current understanding of real-world treatment compliance (adherence and persistence) to BTKis is a knowledge gap. This study aimed to evaluate real-world rates of early adherence and persistence to first-line (1L) ibrutinib or acalabrutinib, for patients with CLL/SLL and further stratified for patients with a diagnosis of baseline atrial fibrillation (AF).
Methods: Adults with CLL/SLL who initiated 1L single-agent ibrutinib or acalabrutinib on or after 11/21/2019, were identified using insurance claims between 11/21/2018-12/31/2021 from Optum's de-identified Clinformatics® Data Mart Database. Patients baseline characteristics were assessed during the 12 months of continuous eligibility (baseline period) prior to the initiation of ibrutinib or acalabrutinib (index date).
Adherence was defined as having a proportion of days covered (PDC) ≥80% for the index treatment (ibrutinib or acalabrutinib) during fixed periods of time of 2, 3, and 6 months during the line of therapy, which lasted until the earliest of second-line treatment initiation, death, or end of continuous enrollment or data. Persistence to 1L treatment with index treatment was defined as not having a gap in treatment >90 days.
The proportion of patients adherent (PDC ≥80%) and persistent to treatment at 2, 3, and 6 months after index were compared between ibrutinib and acalabrutinib using logistic regression models among the overall population, and among those with baseline AF, controlling for the following baseline characteristics: age, gender, 1L regimen (ibrutinib or acalabrutinib), Quan-Charlson Comorbidity Index (Quan-CCI), year of index date, all-cause healthcare costs paid by the patient, total all-cause healthcare costs (patient + payer-paid), and baseline AF.
Results: A total of 542 ibrutinib (mean age: 74.1 years; 43.9% female; mean Quan-CCI: 4.3) and 243 acalabrutinib patients (mean age: 75.1 years; 39.9% female; mean Quan-CCI: 4.2) were included. The median follow-up was 11.8 months for ibrutinib and 9.5 months for acalabrutinib.
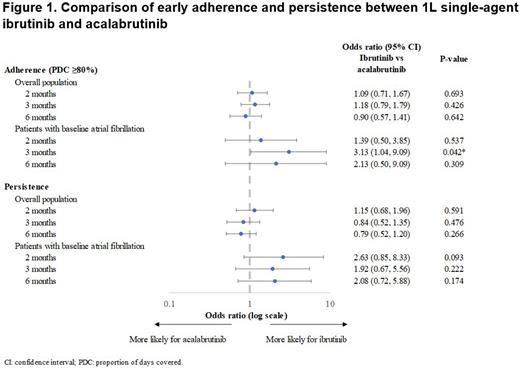
Among the overall population, adherence rates at months 2, 3, and 6 were 84.7%, 81.3%, and 76.3% for ibrutinib and 82.8%, 77.7%, and 76.3% for acalabrutinib. After adjusting for baseline characteristics, adherence was similar across treatment cohorts (Odds ratio [OR] range: 0.9-1.18, all p>.05), as was persistence (OR range: 0.79-1.15, all p>.05) (Figure 1).
Among patients with baseline AF (ibrutinib: N=65; acalabrutinib: N=47), adherence rates at months 2, 3, and 6 were 79.4%, 76.8%, and 72.5% for ibrutinib and 76.6%, 62.5%, and 61.9%, for acalabrutinib. ORs adjusting for baseline characteristics were 1.39 (p=0.537) at 2 months, 3.13 (p=0.042) at 3 months, and 2.13 (p=0.309) at 6 months. Similar results were found for persistence (OR range: 1.92-2.63, all p>.05) (Figure 1).
Conclusions: This study provides insights into real-world adherence and persistence of patients with CLL/SLL initiated on 1L single-agent ibrutinib or acalabrutinib. In this real-world population study, we did not observe greater acalabrutinib treatment compliance in comparison with ibrutinib. On the contrary, for patients with baseline AF, early adherence and persistence rates were higher for ibrutinib than for acalabrutinib. Ibrutinib has dosing advantages due to once-daily administration, lack of interference with concurrent proton pump inhibitors agents, and flexible management guidelines, which may translate into real-world benefits. The current study will further evaluate baseline and post-treatment dose modifications among patients with baseline AF on ibrutinib treatment, and the impact on treatment compliance.
Disclosures
Fradley:Myovant/Pfizer: Consultancy; Medtronic: Research Funding; Takeda: Consultancy; AstraZeneca: Consultancy. Lafeuille:Analysis Group, Inc.: Other: MHL is an employee of Analysis Group, Inc., a consulting company that has provided paid consulting services to Janssen Scientific Affairs, LLC, which funded the development and conduct of this study. Emond:Analysis Group, Inc.: Other: BE is an employee of Analysis Group, Inc., a consulting company that has provided paid consulting services to Janssen Scientific Affairs, LLC, which funded the development and conduct of this study. Crawford:Kite Pharma: Ended employment in the past 24 months; Stratevi: Ended employment in the past 24 months; AbbVie: Current Employment, Other: Family - Current equity holder in private company, Family - Current holder of stock options in a privately held company; Cerner: Current Employment, Other: Family - Current equity holder in private company, Family - Current holder of stock options in a privately held company. Chen:AbbVie: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Volodarsky:Pharmacyclics, an AbbVie Company: Current Employment; AbbVie: Current equity holder in private company, Current holder of stock options in a privately-held company. Nielsen:AbbVie: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Srivastava:AbbVie: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company; Jazz Pharmaceuticals: Current equity holder in private company, Current holder of stock options in a privately-held company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal